Home > Class Action > Medical Device Litigation > Sprint Fidelis Implantable Cardiac Lead Device - Recalled MODELS 6930, 6931, 6948, 6949
Sprint Fidelis Implantable Cardiac Lead Device - Recalled MODELS 6930, 6931, 6948, 6949
 Have you suffered from unnecessary shocks from your defibrillator? If so, your defibrillator may have defective cardiac leads. It is advised that you see your physician as soon as possible. If your leads are recommended for removal or replacement you may be entitled to compensation by the manufacturer. Contact us at no charge to learn your legal options.
Have you suffered from unnecessary shocks from your defibrillator? If so, your defibrillator may have defective cardiac leads. It is advised that you see your physician as soon as possible. If your leads are recommended for removal or replacement you may be entitled to compensation by the manufacturer. Contact us at no charge to learn your legal options.
RECALLED MODELS
Sprint Fidelis® MODEL 6930
Sprint Fidelis® MODEL 6931
Sprint Fidelis® MODEL 6948
Sprint Fidelis® MODEL 6949
Our firm is investigating claims and numerous adverse event reports made to the FDA arising from failures of the Sprint Fidelis Implantable Cardiac Lead, a medical device manufactured by Medtronic, Inc., and its subsidiary, Medtronic Puerto Rico, Inc, These devices are the wires that connect implantable cardiac defibrillators and resynchronization devices to the heart. The Sprint Fidelis Lead may have been used to connect devices manufactured by other companies such as Guidant, Boston Scientific, and St. Jude, as well as Medtronic's own devices. If you have been advised that your ICD lead must be replaced check your device identification card to determine if it is a Medtronic Sprint Fidelis Lead. If so, you may contact us at no charge to discuss your situation.
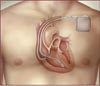
A Lead is part of a implantable heart defibrillation system that has three main parts: the defibrillator, leads, and a programmer. Two parts of this system are placed inside the body.
- The defibrillator is small metal case that contains electronics and a battery. It is similar to a pacemaker in that it is designed to correct arrhythmias. But while a pacemaker increases a slow heart rate, a defibrillator detects and corrects fast and slow heart rates.
- Leads are specialized, thin, insulated wires that are attached to the defibrillator. Leads sense the heart's rhythm and deliver therapy to the heart (as directed by the defibrillator).
The third part, the programmer, is kept in a hospital or clinic. A doctor or nurse uses this specialized computer to monitor and change the instructions of the implanted defibrillator.
If any part of the system fails to function as indicated, a patient is at great risk for serious injury. We have been contacted by individuals whose leads have failed prematurely, leading to replacement. We have also seen a recent rise in reports to the FDA of similar failures.
If you believe that you, your family or friends have been injured by the Sprint Fidelis Lead, please contact Morice Law Firm at (504)-366-1641 or contact us to learn more about the legal options available to you or your loved ones -- FREE of charge.
 Have you suffered from unnecessary shocks from your defibrillator? If so, your defibrillator may have defective cardiac leads. It is advised that you see your physician as soon as possible. If your leads are recommended for removal or replacement you may be entitled to compensation by the manufacturer. Contact us at no charge to learn your legal options.
Have you suffered from unnecessary shocks from your defibrillator? If so, your defibrillator may have defective cardiac leads. It is advised that you see your physician as soon as possible. If your leads are recommended for removal or replacement you may be entitled to compensation by the manufacturer. Contact us at no charge to learn your legal options.